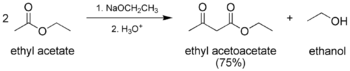
Acetoacetic-Ester Condensation Claisen Condensation
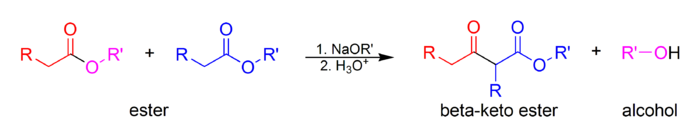
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887.
Requirements
At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen condensations.
The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the alkoxide is regenerated. In mixed Claisen condensations, a non-nucleophilic base such as lithium diisopropylamide, or LDA, may be used, since only one compound is enolizable. LDA is not commonly used in the classic Claisen or Dieckmann condensations due to enolization of the electrophilic ester.
The alkoxy portion of the ester must be a relatively good leaving group. Methyl and ethyl esters, which yields methoxide and ethoxide, respectively, are commonly used.
Types
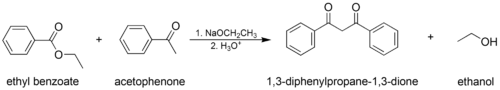
- The classic Claisen condensation, a self-condensation between two molecules of a compound containing an enolizable ester.
- The mixed (or "crossed") Claisen condensation, where one enolizable ester or ketone and one nonenolizable ester are used.
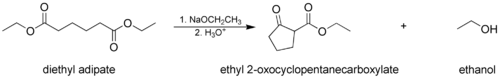
- The Dieckmann condensation, where a molecule with two ester groups reacts intramolecularly, forming a cyclic β-keto ester. In this case, the ring formed must not be strained, usually a 5- or 6-membered chain or ring.

In the first step of the mechanism, an α-proton is removed by a strong base, resulting in the formation of an enolate anion, which is made relatively stable by the delocalization of electrons. Next, the carbonyl carbon of the (other) ester is nucleophilically attacked by the enolate anion. The alkoxy group is then eliminated (resulting in (re)generation of the alkoxide), and the alkoxide removes the newly formed doubly α-proton to form a new, highly resonance-stabilized enolate anion. Aqueous acid (e.g. sulfuric acid or phosphoric acid) is added in the final step to neutralize the enolate and any base still present. The newly formed β-keto ester or β-diketone is then isolated. Note that the reaction requires a stoichiometric amount of base as the removal of the doubly α-proton thermodynamically drives the otherwise endergonic reaction. That is, Claisen condensation does not work with substrates having only one α-hydrogen because of the driving force effect of deprotonation of the β-keto ester in the last step.
 |
| animation |
Stobbe condensation
The Stobbe condensation is a modification specific for the diethyl ester of succinic acid requiring less strong bases. An example is its reaction with benzophenone:
A reaction mechanism that explains the formation of both an ester group and a carboxylic acid group is centered on a lactone intermediate :
Literature References
1- General, Robust, and Stereocomplementary Preparation of α,β-Disubstituted α,β-Unsaturated EstersHidefumi Nakatsuji, Hiroshi Nishikado, Kanako Ueno and Yoo Tanabe*
*Department of Chemistry, School of Science and Technology, Kwansei Gakuin University, 2-1 Gakuen, Sanda, Hyogo 669-1337, Japan, Email: tanabe kwansei.ac.jp
kwansei.ac.jp
 kwansei.ac.jp
kwansei.ac.jp
H. Nakatsuji, H. Nishikado, K. Ueno, Y. Tanabe, Org. Lett., 2009, 11, 4258-4261.
DOI: 10.1021/ol9013359 (free Supporting Information)

2- Direct Carbon-Carbon Bond Formation via Chemoselective Soft Enolization of Thioesters: A Remarkably Simple and Versatile Crossed-Claisen Reaction Applied to the Synthesis of LY294002Guoqiang Zhou, Daniel Lim and Don M. Coltart*
*Department of Chemistry, Duke University, Durham, North Carolina 27708, Email: don.coltart duke.edu
duke.edu
 duke.edu
duke.edu
G. Zhou, D. Lim, D. M. Coltart, Org. Lett., 2008, 10, 3809-3812.
DOI: 10.1021/ol801498u (free Supporting Information)

Abstract
Thioesters undergo chemoselective soft enolization and acylation by N-acylbenzotriazoles on treatment with MgBr2·OEt2 and i-Pr2NEt to give β-keto thioesters without prior enolate formation. The reaction is conducted using untreated CH2Cl2 open to the air. The coupled products can be converted directly into β-keto esters, β-keto amides, and β-diketones under mild conditions.

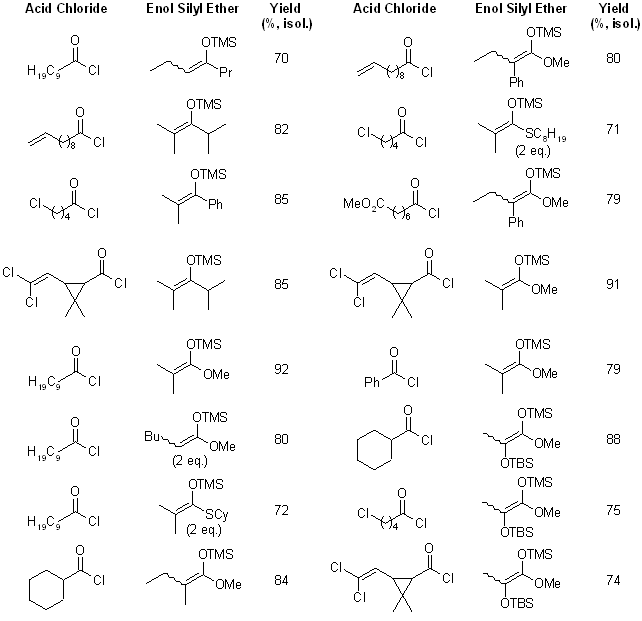
3- Mild and Efficient Pentafluorophenylammonium Triflate (PFPAT)-Catalyzed C-Acylations of Enol Silyl Ethers or Ketene Silyl (Thio)Acetals with Acid ChloridesAkira Iida, Jun Osada, Ryohei Nagase, Tomonori Misaki and Yoo Tanabe*
*Department of Chemistry, School of Science and Technology, Kwansei Gakuin University, 2-1 Gakuen, Sanda, Hyogo 669-1337, Japan, Email: tanabe kwansei.ac.jp
kwansei.ac.jp
 kwansei.ac.jp
kwansei.ac.jp
A. Iida, J. Osada, R. Nagase, T. Misaki, Y. Tanabe, Org. Lett., 2007, 9, 1859-1862.
DOI: 10.1021/ol070191b (free Supporting Information)

Abstract
Pentafluorophenylammonium triflate (PFPAT) successfully catalyzed the C-acylation of enol silyl ethers with acid chloride to produce various β-diketones in good yield. Similarly, C-acylation of ketene silyl acetals or ketene silyl thioacetals proceeded smoothly to provide also thermodynamically unfavorable α,α-dialkylated β-keto (thio)esters in good yield.

Betfair Casino Review & Bonus Code - KTM Hub
ReplyDeleteBetfair Casino 상주 출장안마 Review. 안동 출장샵 Betfair Casino Bonus Codes 2021. Check out the top Betfair Casino offers from Top Betfair. Deposit 제천 출장안마 & withdraw 김포 출장샵 with promo code Rating: 8.3/10 · Review by JT Hub 동해 출장샵