Reaction Procedure of Appel Reaction & Workup
Org. Synth. 1974, 54, 63
DOI: 10.15227/orgsyn.054.0063
Example 1:-
GERANYL CHLORIDE
[2,6-Octadiene, 1-chloro-3,7-dimethyl-, (E)-]
 |
1. Procedure
A dry, 300-ml., three-necked flask is equipped with a magnetic stirring bar and reflux condenser (to which is attached a Drierite-filled drying tube) and charged with 90 ml. of carbon tetrachloride (Note 1) and 15.42 g. of geraniol(0.1001 mole) (Note 2). To this solution is added 34.09 g. (0.1301 mole) of triphenylphosphine (Note 3), and the stirred reaction mixture is heated under reflux for 1 hour. This mixture is allowed to cool to room temperature; dry pentane is added (100 ml.), and stirring is continued for an additional 5 minutes.
The triphenylphosphine oxide precipitate is filtered and washed with 50 ml. of pentane. The solvent is removed from the combined filtrate with a rotary evaporator under water aspirator pressure at room temperature. Distillation of the residue through a 2-cm. Vigreux column attached to a short-path distillation apparatus (Note 4) provides 13.0–14.0 g. (75–81%) of geranyl chloride, b.p. 47–49° (0.4 mm.), n23D = 1.4794 (Note 5).
2. Notes
1. Carbon tetrachloride was dried over magnesium sulfate and distilled from phosphorus pentoxide through a 25-cm. Vigreux column. Lower yields were obtained when either the glassware or reagents were not dried.
2. Geraniol was purchased from Koch-Light Laboratories (>98% pure), dried over potassium carbonate and distilled through an 8-cm. Vigreux column, b.p. 108–109° (8 mm.). The checkers used geraniol purchased from Aldrich Chemical Co., Inc., and distilled it prior to use.
3. Triphenylphosphine, m.p. 80–81°, was obtained from Eastman Organic Chemicals and kept in a drying pistol held at approximately 65° (1 mm.) for 18 hours prior to use. When only 10–20% excess triphenylphosphine was employed the yield of geranyl chloride was approximately 75%, but the small amount of unreacted geraniol which remained rendered product isolation more difficult.
4. A “Bantam-ware” distilling head and condenser assembly from Kontes Glass Co. (K-287100) was used. Foaming may occur due to incomplete removal of solvent. This can be avoided by cooling the distillation flask to approximately −50° and gradually lowering the pressure to 10 mm. The pot temperature is then allowed to increase gradually to room temperature, and the distillation then proceeds without difficulty.
5. The pure geranyl chloride has characteristic IR absorption (liquid film) at 845, 1255, and 1665 cm.−1. The use of these absorptions to assay mixtures of geranyl and linalyl chloride has been discussed in detail.2 The 1H NMR spectrum (100 MHz., CCl4) shows absorption at δ 1.61 and 1.67 [2s, 6H, C=C(CH3)2], 1.71 [d, J = 1.4 Hz., 3H, C=C(CH3CH2], 2.05 (m, 4H, 2CH2), 3.98 (d, J = 8 Hz., 2H, CH2Cl), 5.02 [m, 1H, CH=C(CH3)2], and 5.39 (t of partially resolved m, 1H, C=CHCH2Cl).
Working with Hazardous Chemicals
The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
The paragraphs above were added in September, 2014. The statements above do not supersede any specific hazard caution notes and safety instructions included in the procedure.
3. Discussion
Geranyl chloride has been prepared by allylic rearrangement of linaloolusing hydrogen chloride in toluene solution at 100° or phosphorus trichloride in the presence of potassium carbonate at 0°. The conversion of geraniol to geranyl chloride has been reported using hydrogen chloride in toluene,phosphorus trichloride or phosphorus pentachloride in petroleum ether, and thionyl chloride and pyridine. These methods give mixtures of geranyl and linalyl chloride, which are difficult to separate; tedious fractionation is required to isolate the geranyl chloride. Procedures which give a pure product involve treatment of geraniol (a) in ether–hexamethylphosphoric triamide(HMPA) with methyllithium, followed by p-toluenesulfonyl chloride and lithium chloride in ether–HMPA; (b) in 2,4,6-collidine with lithium chloride in N,N-dimethylformamide, followed by methanesulfonyl chloride; and (c) in pentane with methanesulfonyl chloride at −5°, followed by the addition of pyridine.
The present procedure is representative of a fairly general method of converting alcohols to chlorides using carbon tetrachloride and a tertiary phosphine. The reaction occurs under mild, essentially neutral conditions and, as illustrated by the present synthesis, may be employed to convert allylic alcohols to the corresponding halide without allylic rearrangement.
Carbon tetrachloride serves as both solvent and halogen source. Several trivalent phosphorus reagents may be employed, including triphenylphosphine, tri-n-octylphosphine, tri-n-butylphosphine, and trisdimethylaminophosphine. The latter “more nucleophilic” phosphines react more rapidly and under milder conditions than triphenylphosphine. When triphenylphosphine is employed, the by-product, triphenylphosphine oxide, usually precipitates completely and is easily removed by filtration. After evaporation of the solvent, the product is isolated in high purity by distillation. On occasion, difficulties may be encountered in separating the alkyl halide from the accompanying oxide. The presence of residual soluble organophosphine oxide may pose a serious problem when attempting to isolate sensitive (e.g., allylic or optically active) halides and can lead to loss of product, racemization, etc. This difficulty is usually resolved, as illustrated in the present synthesis, by adding a diluent such as pentane to ensure precipitation. Although precise conditions will undoubtedly depend on the specific substrate at hand, it is usually desirable to employ a modest excess of the organophosphine. This is especially helpful for the preparation of sensitive halides since, by ensuring complete consumption of alcohol, it simplifies the isolation procedure (Note 3)by avoiding the possible necessity of a careful fractionation step.
Triphenylphosphine reacts with carbon tetrachloride or carbon tetrabromide in the absence of alcohols, forming the corresponding triphenylphosphine dihalomethylene ylide and triphenylphosphine dihalide.
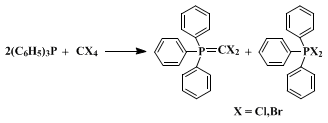
The mechanism has been viewed as involving either the formation of a pentacovalent phosphorus intermediate, or alternatively, by initial nucleophilic attack on halogen, forming an intermediate phosphonium species.These mixtures react with carbonyl compounds, providing a useful route to 1,1-dihaloalkenes.
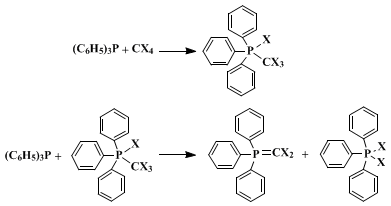
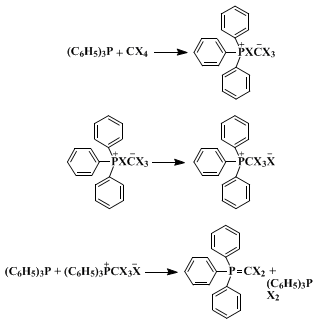
The success of the present method depends critically on the initial presence of an alcohol to trap the intermediate phosphonium species. If the alcohol is added last, the R3P-CX4 reaction described above (an exothermic process for the more nucleophilic phosphines) may go to completion, in which case little or no alkyl halide is formed. Since the reaction displays several characteristics of an SN2 process, it is thought to proceed by the pathway illustrated:
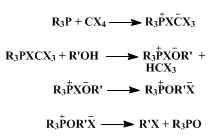
Yields of chlorides are good to excellent for primary and secondary alcohols, but a competing olefin-forming elimination process renders the method of limited value for preparing tertiary chlorides. An adaptation of the procedure using carbon tetrabromide allows the synthesis of alkyl bromides. Some examples are the preparation of n-pentyl bromide (97%) and benzyl bromide (96%). Farnesyl bromide has been prepared in 90% yield from farnesol.
The advantages of the carbon tetrahalide–organophosphine–alcohol preparation of halides are simplicity of experimental procedure, good yields, relatively mild, essentially neutral reaction conditions, and absence of allylic rearrangements. The reaction proceeds with inversion of configuration and is a useful simple device for converting optically active alcohols to chiral halides in high optical purity.
This preparation is referenced from:
References and Notes
- Department of Chemistry, University of Alberta, Edmonton, Alberta, Canada. T6G 2G2.
- D. Barnard, L. Bateman, A. J. Harding, H. P. Koch, N. Sheppard, and G. B. B. M. Sutherland, J. Chem. Soc., 915 (1950).
- J. L. Simonsen, and L. N. Owen, “The Terpenes,” Vol. I, 2nd ed., Cambridge University Press, 1953, pp. 50, 63.
- L. Ruzicka, Helv. Chim. Acta, 6, 492 (1923).
- M. O. Forster, and D. Cardwell, J. Chem. Soc., 1338 (1913).
- D. Barnard, and L. Bateman, J. Chem. Soc., 926 (1950).
- R. M. Carman, and N. Dennis, Aust. J. Chem., 20, 783 (1967).
- C. A. Bunton, D. L. Hachey, and J.-P. Leresche, J. Org. Chem., 37, 4036 (1972).
- G. Stork, P. A. Grieco, and M. Gregson, Tetrahedron Lett., 1393 (1969); Org. Synth., Coll. Vol. 6, 638 (1988).
- E. W. Collington, and A. I. Meyers, J. Org. Chem., 36, 3044 (1971).
- I. M. Downie, J. B. Holmes, and J. B. Lee, Chem. Ind. (London), 900 (1966); J. B. Lee, and T. J. Nolan, Can. J. Chem., 44, 1331 (1966).
- J. Hooz, and S. S. H. Gilani, Can. J. Chem., 46, 86 (1968).
- I. M. Downie, J. B. Lee, and M. F. S. Matough, Chem. Commun., 1350 (1968).
- R. Rabinowitz, and R. Marcus, J. Am. Chem. Soc., 84, 1312 (1962).
- F. Ramirez, N. B. Desai, and N. McKelvie, J. Am. Chem. Soc., 84, 1745 (1962).
- B. Miller, in M. Grayson, and E. J. Griffith, Eds. “Topics in Phosphorus Chemistry,” Vol. 2, Wiley-Interscience, New York, 1965, p. 133.
- Reagents of the type R3PX2 (R=C4H5, n-C4H9, OC6H5) have independently been employed to convert alcohols to halides and phenols to aryl halides.18,19,20 The reaction of (C6H5O)3P with methyl iodide and an alcohol gives good yields of iodides. Replacing the methyl iodide by benzyl bromide or chloride permits the synthesis of alkyl bromides and chlorides.21
- L. Horner, H. Oediger, and H. Hoffmann, Justus Liebigs Ann. Chem., 626, 26 (1959).
- H. Hoffmann, L. Horner, H. G. Wippel, and D. Michael, Chem. Ber., 95, 523 (1962).
- G. A. Wiley, R. L. Hershkowitz, B. M. Rein, and B. C. Chung, J. Am. Chem. Soc., 86, 964 (1964).
- H. N. Rydon, Org. Synth., Coll. Vol. 6, 830 (1988).
- E. H. Axelrod, G. M. Milne, and E. E. van Tamelen, J. Am. Chem. Soc., 92, 2139 (1970).
- D. Brett, I. M. Downie, J. B. Lee, and M. F. S. Matough, Chem. Ind. (London), 1017 (1969).
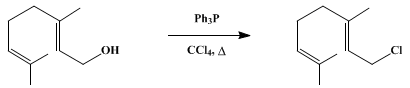
Example :-2
General Characteristics
The Appel reaction converts primary and secondary alcohols into corresponding alkyl halides. The reaction is suitable for both acid- and base-sensitive alcohols since the reaction conditions are neutral.
Reaction Mechanism
The reaction is driven based on the high affinity between phosphorus and oxygen.

Examples
The substitution involves the inversion of stereochemistry. [1]

The Appel reaction proceeds at very low temperatures under reactive conditions using hexachloroacetone[2a] or hexabromoacetone.[2b] These can be used for even some tertiary alcohols including adamantanol.
Asymmetric phosphine oxidation under the Appel conditions. [3]

Modified conditions in which the phosphine can be used catalytically have been developed recently.[4]

Experimental Procedure
The synthesis of geranyl chloride.[4]

Experimental Tips
-Bromination with CBr4 proceeds at 0˚C~room temperature, but chlorination with CCl4 requires reflux temperature.
-The separation of phosphine oxide from the product can be a hassle. An alternative strategy to effect the same transformation would be the Finkelstein reaction, which is a two-step reaction involving mesylation followed by halide displacement.
References
[2] (a) Magid, M. R.; Fruchey, S.; Johnson, W. L.; Allen, T. G. J. Org. Chem. 1979, 44, 359. DOI: 10.1021/jo01317a011 (b) Tongkatea, P.; Pluempanupata, W.; Chavasiri, W. Tetrahedron Lett. 2008, 49, 1146. doi:10.1016/j.tetlet.2007.12.061
[3] Bergin, E.; O’Connor, C. T.; Robinson, S. B.; McGarrigle, E. B.; O’Mahony, C. P.; Gilheany, D. G. J. Am. Chem. Soc. 2007, 129, 9566. DOI:10.1021/ja072925l
[4] (a) Denton, R.; An, J.; Adeniran, B.; Blake, A.; Lewis, W.; Poulton, A. J. Org. Chem. 2011, 76, 6749. doi:10.1021/jo201085r (b) van Kalkeren, H. A.; Leenders, S. H. A. M.; Hommersom, C. A.; Rutjes, F. P. J. T.; van Delft, F. L. Chem. Eur. J. 2011, 17, 11290. DOI: 10.1002/chem.201101563
[5] Calzada, J. G.; Hooz, J. Org. Synth. 1974, 54, 63. [PDF]
Some other Literature & Patent references of performing reaction and their workup procedure
http://irserver.ucd.ie/bitstream/handle/10197/4936/RajendranGilheany_U-turn.pdf?sequence=1
http://www.usc.es/congresos/ecsoc/15/hall_a_GOS/a002/index.pdf
http://researchrepository.ucd.ie/bitstream/handle/10197/4940/ByrneRajendranGilheanyOBCMSFinalV7.pdf?sequence=1
http://www.rsc.org/suppdata/cc/c0/c002825h/c002825h.pdf
http://dspace.uah.es/dspace/bitstream/handle/10017/3581/468.pdf?sequence=1
https://www.scripps.edu/shenvi/Education_files/work%20up%20tips.pdf
Comments
Post a Comment