Arbuzov Reaction Michaelis-Arbuzov Reactio
The Michaelis–Arbuzov reaction (also called the Arbuzov reaction) is the chemical reaction of a trialkyl phosphite and an alkyl halide to form a phosphonate.

The reaction was discovered by August Michaelis in 1898, and greatly explored by Aleksandr Arbuzov soon thereafter. This reaction is widely used for the synthesis of various phosphonates, phosphinates, and phosphine oxides. Several reviews have been published.The reaction also occurs for coordinated phosphite ligands, as illustrated by the demethylation of {(C5H5)Co[(CH3O)3P]3}2+ to give {(C5H5)Co[(CH3O)2PO]3}−, which is called the Klaui ligand.

Reaction mechanism
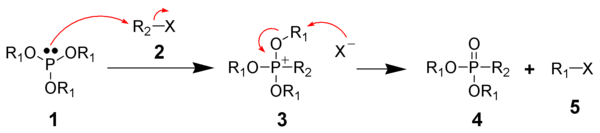
The Michaelis–Arbuzov reaction is initiated with the SN2 reaction of the nucleophilic phosphite (1) with the electrophilic alkyl halide (2) to give a phosphonium intermediate (3). Triaryl phosphites, which are unable to perform the second step of the Michaelis–Arbuzov reaction, have been shown to produce stable phosphonium salts.[6] Likewise, aryl and vinyl halides are less reactive towards phosphites.
The displaced halide anion reacts via another SN2 reaction with the phosphonium intermediate to give the desired phosphonate (4) and another alkyl halide (5). When chiral phosphonium intermediates are produced, it has been shown the halide substitution proceeds with inversion of configuration, as expected by a SN2 reaction.[7]
As a general guideline, the reactivity of the organic halide (2) can be listed as follows: (from most reactive to least reactive)
and
The reaction of α-bromo- and α-chloroketones with phosphites yields a vinyl phosphate instead of an alkyl phosphonate – the Perkow reaction. α-Iodoketones do, in fact, give the a phosphonate.[8] Other methods of producing β-ketophosphonates have been developed.
Recent Literature

Lewis Acid-Mediated Michaelis-Arbuzov Reaction at Room Temperature: A Facile Preparation of Arylmethyl/Heteroarylmethyl Phosphonates
G. G. Rajeshwaran, M. Nandakumar, R. Sureshbabu, A. K. Mohanakrishnan, Org. Lett., 2011, 13, 1270-1273.

Generation of Phosphoranes Derived from Phosphites. A New Class of Phosphorus Ylides Leading to High E Selectivity with Semi-stabilizing Groups in Wittig Olefinations
V. K. Aggarwal, J. R. Fulton, C. G. Sheldon, J. de Vicente, J. Am. Chem. Soc., 2003, 125, 6034-6035.
Reliable and Versatile Synthesis of 2-Aryl-Substituted Cinnamic Acid Esters
A. Ianni, S. R. Waldvogel, Synthesis, 2006, 2103-2112.

Lewis Acid-Mediated Michaelis-Arbuzov Reaction at Room Temperature: A Facile Preparation of Arylmethyl/Heteroarylmethyl Phosphonates
G. G. Rajeshwaran, M. Nandakumar, R. Sureshbabu, A. K. Mohanakrishnan, Org. Lett., 2011, 13, 1270-1273.

Generation of Phosphoranes Derived from Phosphites. A New Class of Phosphorus Ylides Leading to High E Selectivity with Semi-stabilizing Groups in Wittig Olefinations
V. K. Aggarwal, J. R. Fulton, C. G. Sheldon, J. de Vicente, J. Am. Chem. Soc., 2003, 125, 6034-6035.
Reliable and Versatile Synthesis of 2-Aryl-Substituted Cinnamic Acid Esters
A. Ianni, S. R. Waldvogel, Synthesis, 2006, 2103-2112.
Want other example click the link
http://www.google.co.in/patents/US3483279


Comments
Post a Comment